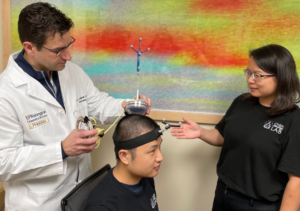
FDA grants WashU-based technology ‘Breakthrough Device’ designation A device aimed at enabling neurosurgeons and other physicians to perform noninvasive blood-based biopsies in adults with brain tumors has received Food and Drug Administration (FDA) “Breakthrough Device” designation. The device includes technology from Washington University in St. Louis and developed by Cordance Medical Inc., a medical device […]
Device for noninvasive brain biopsies via blood draw moves closer to market approval