FDA grants WashU-based technology ‘Breakthrough Device’ designation
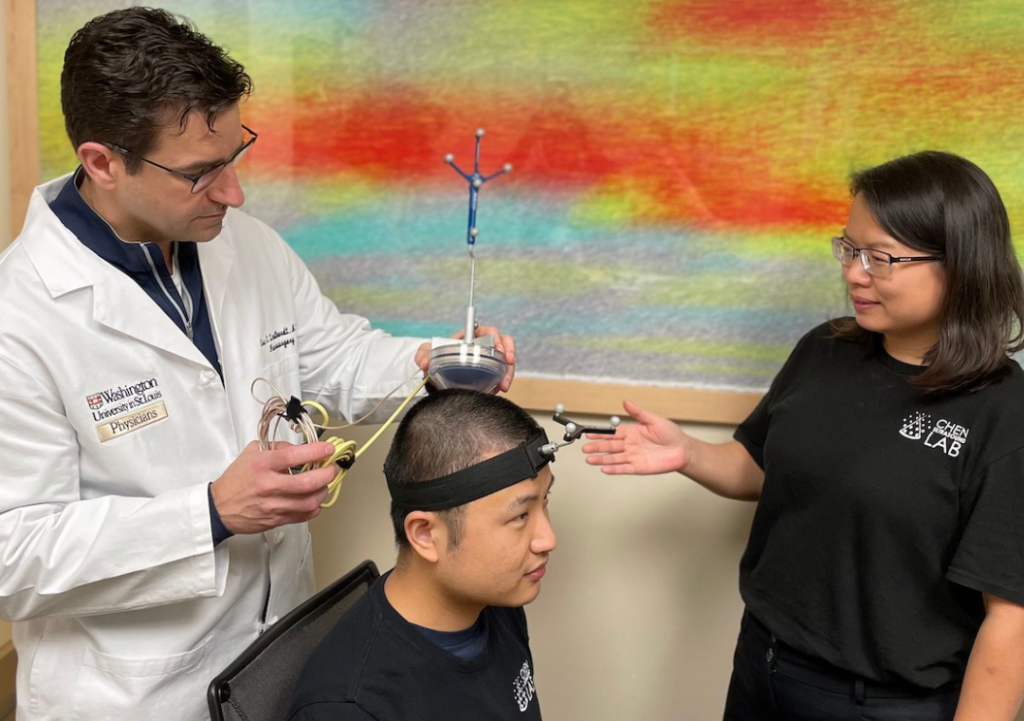
A device aimed at enabling neurosurgeons and other physicians to perform noninvasive blood-based biopsies in adults with brain tumors has received Food and Drug Administration (FDA) “Breakthrough Device” designation. The device includes technology from Washington University in St. Louis and developed by Cordance Medical Inc., a medical device company in Mountain View, Calif.
The designation is aimed at providing patients and health-care providers with timely access to novel medical devices by expediting the review process needed to bring technology toward market approval.
The FDA distinction applies to devices that have undergone rigorous review and show exceptional promise of improved treatment or the ability to diagnose life-threatening or debilitating diseases. The technology was co-invented by Eric C. Leuthardt, MD, the Shi Hui Huang Professor of Neurosurgery at Washington University School of Medicine, and by Hong Chen, PhD, an associate professor of biomedical engineering at the university’s McKelvey School of Engineering and of neurosurgery at the School of Medicine.
Read the full story here.